Melissa Laitner
Emily Shambaugh
Katherine Bowman
Background Information
Advances in biomedical research, data science, engineering, and technology are leading to high-speed innovation with tremendous potential to transform health and medicine. At the same time, these innovations carry potential risks and important societal implications related to access, ethics, and public trust. There is an urgent need to understand, anticipate, and respond to the effects of such advances.
In this context, the National Academy of Medicine (NAM) hosted a workshop in May 2024 entitled “Fostering Action to Address Ethical and Societal Implications of Emerging Science, Technology, and Innovation in Health and Medicine” (see Box 1 for a list of workshop planning committee members and Box 2 for a list of speakers and moderators). The workshop included discussions to highlight past and ongoing efforts led by the NAM to align emerging technologies with ethical principles through effective governance, aiming to build consensus and momentum on actionable steps moving forward.
Box 1 | Workshop Planning Committee Members
Victor Dzau, MD (Chair), President, National Academy of Medicine
Tim Persons, PhD, MS, Artificial Intelligence Leader, PricewaterhouseCoopers
Jay J. Schnitzer, MD, PhD, Senior Vice President, Corporate Chief Engineer, and Chief Medical Officer, MITRE
Hana El-Samad, PhD, Senior Vice President and Director, Institute of Computation, Altos Labs
Anthony Ryan Hatch, PhD, MA, Professor, Wesleyan University
Alex John London, PhD, Professor, Carnegie Mellon University
Shobita Parthasarathy, PhD, Professor, University of Michigan
Juan Enriquez, MBA, Author, Business Leader, and Affiliate, Massachusetts Institute of Technology Synthetic Neurobiology Lab
SOURCE: Created by authors.
NOTE: Affiliations listed reflect individual institutional positions at the time of the workshop.
Box 2 | Workshop Speakers and Moderators
Monica M. Bertagnolli, MD, Director, National Institutes of Health
Robert M. Califf, MD, MACC, Commissioner, U.S. Food and Drug Administration
Rep. Bill Foster, PhD, U.S. Congressman (IL-11) [a]
Alondra Nelson, PhD, Harold F. Linder Professor, Institute for Advanced Study
David Winickoff, JD, MA, Head of Responsible Innovation Unit, Organisation for Economic Co-Operation and Development
Debra JH Mathews, PhD, MA, Associate Director for Research and Programs and Professor, Johns Hopkins Berman Institute of Bioethics
Keith Wailoo, PhD, Henry Putnam University Professor, Princeton University
William “Bill” Dietz, PhD, Director of Research and Policy for the Global Food Institute, Director of STOP Obesity Alliance, and Professor, Department of Exercise and Nutritional Sciences, George Washington University, Milken Institute School of Public Health
Joseph “Joe” Nadglowski, President and Chief Executive Officer, Obesity Action Coalition
Michael G. Knight, MD, MSHP, FACP, Dipl. ABOM, Head of Services, IMPaCT Care and Clinical Associate Professor of Medicine, George Washington University
Shiriki Kumanyika, PhD, MS, Emeritus Professor, University of Pennsylvania and Research Professor, Drexel University
Lynda Stuart, MD, PhD, Professor of Practice, University of Washington [b]
Madeleine Clare Elish, PhD, Head of Responsible Artificial Intelligence, Google Cloud
Garth Graham, MD, MPH, FACC, Director and Global Head, Healthcare and Public Health, Google/YouTube
Lori Melichar, PhD, MA, Senior Director, Robert Wood Johnson Foundation
George Q. Daley, MD, PhD, Dean, Harvard Medical School
Keith Yamamoto, PhD, Vice Chancellor for Science Policy, University of California, San Francisco
Santa J. Ono, PhD, President, University of Michigan
Patricia “Patti” Mae Doykos, PhD, Executive Director for Global Health Equity, Bristol Myers Squibb
Paula T. Hammond, PhD, Institute Professor, Massachusetts Institute of Technology
Christopher A. Viehbacher, President and Chief Executive Officer, Biogen
Eric Rubin, MD, PhD, Editor-in-Chief, New England Journal of Medicine
Holden Thorp, PhD, Editor-in-Chief, Science journals and Professor, George Washington University
Gil Omenn, MD, PhD, Professor, University of Michigan
Samsher “Sam” Singh Gill, President and Chief Executive Officer, Doris Duke Foundation
Eliseo Pérez-Stable, MD, Director, National Institute on Minority Health and Health Disparities, National Institutes of Health
Holly Fernandez Lynch, JD, MBE, Assistant Professor, University of Pennsylvania
Susan Coller Monarez, PhD, Deputy Director, Advanced Research Projects Agency for Health
Erica Kimmerling, PhD,Assistant Director for Community Driven Health, White House Office of Science and Technology Policy
SOURCE: Created by authors.
NOTE: Affiliations listed reflect individual institutional positions at the time of the workshop. [a] Representative Foster is the only PhD-holding physicist in Congress. [b] Stuart was formerly employed by the Gates Foundation and BioNTech.
Meeting Summary
Context and Framing
The workshop began with framing remarks provided by Dr. Victor Dzau, National Academy of Medicine, and key government officials with jurisdiction across federal science, technology, and health domains.
In his opening remarks, Dr. Dzau emphasized that no single entity can comprehensively govern the complex and rapidly evolving landscape of biomedical innovation, nor the tools and techniques it generates. Dr. Dzau observed that the swift advancement and cross-boundary diffusion of technologies require a coordinated, dynamic approach to governance and that the innovation ecosystem encompasses a wide range of stakeholders, including funders, researchers, developers, publishers, investors, regulators, health care organizations, payers, and users—including patients and affected communities. He highlighted that each participant, through their decisions and actions, can shape the trajectory of these technologies and influence the distribution of their benefits and burdens. Dr. Dzau said that levers exist to align innovations with ethical principles at every stage of the innovation life cycle (see Figure 1).
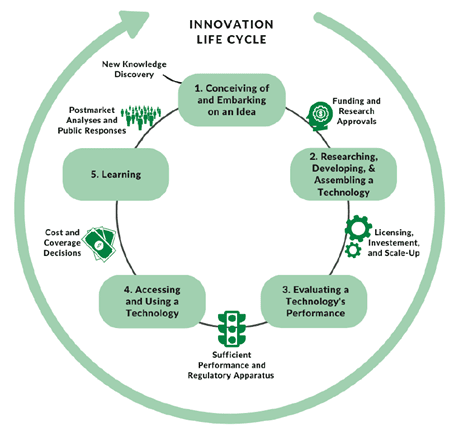
Figure 1 | A Basic Conceptual Framework of the Innovation Life Cycle SOURCE: NASEM and NAM. 2023. Toward equitable innovation in health and medicine: A framework. Washington, DC: The National Academies Press. https://doi.org/10.17226/27184. NOTE: The figure highlights that innovation in health care and medicine is a multifaceted and dynamic process involving diverse contributors; allowing for repeated transitions within and across different stages; and including iterative loops of research, development, assessment, and learning.
After Dr. Dzau’s introduction, government officials offered additional framing comments. Dr. Monica Bertagnolli, National Institutes of Health (NIH), emphasized the importance of including representative patient communities in scientific research. While foundational research remains essential, she mentioned that the NIH is equally committed to ensuring that discoveries are translated into real-world applications that positively impact individuals. Dr. Bertagnolli reaffirmed her commitment, as NIH Director, to partnering with government and health care stakeholders to promote access to medical innovations. Dr. Robert Califf, US Food and Drug Administration, addressed the need to democratize health care access, raising concerns about declining health in the United States. Despite leading in technological advancements, the United States ranks 34th globally in health outcomes, with projections indicating further decline by 2035 (United Health Foundation, 2014). Dr. Califf stressed that lower-income countries are achieving better health outcomes and surpassing the United States by widening margins, underscoring the urgent need for systemic improvements in American health care (Emanuel et al., 2020). Congressman Bill Foster (D-IL) emphasized the transformative potential of technology, particularly artificial intelligence (AI), in expanding access to health care and information. However, he pointed out, achieving these benefits requires overcoming budget constraints and ensuring widespread access to these technologies. Congressman Foster underscored the need for incentives that steer private sector innovation toward addressing key public health priorities, cautioning that a purely profit-driven approach could result in unintended health consequences.
Dr. Alondra Nelson, Institute for Advanced Study, then provided the workshop’s keynote address. Dr. Nelson stressed the importance of both dynamic governance for emerging technologies and the involvement of diverse public and private partners in their development. She then highlighted the essential role of US government stewardship and funding in shaping the nation’s innovation ecosystem. Dr. Nelson credited this stewardship—including the creation of the National Science Foundation; the continued support of funding agencies; and the individual efforts of federally funded institutional review boards (IRBs), peer reviewers, and bioethics experts—as foundational for cultivating the culture and breakthroughs of 20th-century scientific research. She observed a recent departure from this type of government leadership, as the private sector has increasingly assumed a larger role in research and innovation over the past two decades. Dr. Nelson noted that this change has rendered some of these guiding mechanisms outdated and called for a refreshed approach to governance and oversight of emerging technologies. Dr. Nelson explained that the research and development (R&D) landscape is shifting as the private sector’s influence grows, including how government funding for R&D dropped from 30 percent in 2011 to 19 percent in 2021, while industry now funds nearly 36 percent of basic research (NSF NSB, 2024). Dr. Nelson emphasized the continued importance of the public sector in the evolving landscape of emerging technologies. She suggested that policies should be designed to ensure that public investments yield public benefits, citing economist Maria Mazzucato (Mazzucato, 2013). Dr. Nelson suggested that beyond merely providing funding, government stakeholders could make strategic investments and decisions that shape market outcomes, drive innovation, and foster social benefits. Additionally, she noted, the government can accelerate discovery and impact by partnering with the private sector, philanthropic organizations, academic and clinical communities, and community and patient organizations. Dr. Nelson discussed the Creating Helpful Incentives to Produce Semiconductors and Science Act of 2022 as a prime example of federal commitment to research, innovation, and manufacturing (US Congress, 2022).
Dr. Nelson then highlighted AI-enabled tools as examples of rapidly advancing health and medical technologies that may fall short of their potential without intentional efforts to promote responsible use. She argued that new approaches to oversight are essential, given the dynamic evolution of AI applications, and emphasized the need to move beyond one-time reviews to a model that includes regular, proactive reevaluation. Dr. Nelson pointed to several best practices for the design and assessment of AI-based tools, many of which were drawn from private sector solutions to mitigate hazards, including risk assessments, auditing mechanisms, assessment of organizational procedures, dashboards for ongoing monitoring, and more. Dr. Nelson noted that these solutions informed the White House Office of Science and Technology Policy (OSTP)’s 2022 Blueprint for an AI Bill of Rights: Making Automated Systems Work for the American People, but stressed that such measures remain mostly voluntary (OSTP, 2022). Effective governance, she argued, must weigh both the benefits and risks of emerging technologies and work to align technical capabilities with positive public outcomes in real time.
To introduce the concept that innovation in health and medicine operates within a global governance ecosystem, Mr. David Winickoff, Organisation for Economic Co-operation and Development (OECD), stressed the importance of an international approach to governing emerging technologies to ensure coordination and harmonization across countries, multilateral organizations, and private entities. He advocated for a “technology-agnostic framework” to address recurring challenges like balancing risks and benefits amid uncertainty, adapting governance to the novel and disruptive integration of technologies, and fostering cooperation within a multilateral system in a competitive global landscape (NATO Science & Technology Organization, 2020).
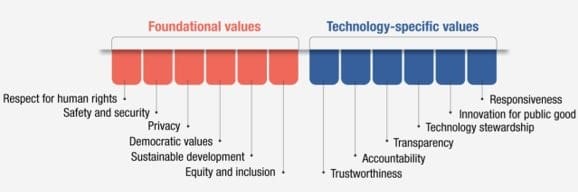
Figure 2 I Foundational Values and Technology Governance-Specific Values
SOURCE: OECD. 2024. Framework for anticipatory governance of emerging technologies. https://doi.org/10.1787/0248ead5-en.
In 2024, OECD introduced its Framework for Anticipatory Governance of Emerging Technologies, which was designed to encourage responsible innovation across various policy fields. This framework emphasizes “shared values, anticipation, societal engagement, agile governance, and international cooperation” (OECD, 2024; see Figure 2 for additional information on values). Mr. Winickoff pointed to an international policy database and an international expert network on agile platforms as initiatives that could facilitate knowledge exchange and partnerships, including with the private sector, to support equitable innovation frameworks worldwide.
History of NAM Efforts
Dr. Dzau, along with Dr. Debra Mathews, Johns Hopkins Berman Institute of Bioethics, and Dr. Keith Wailoo, Princeton University, led an in-depth discussion on past NAM initiatives regarding the state of the innovation ecosystem and how to apply equity principles to such work. They reviewed NAM’s prior efforts and presented a framework for evaluating case studies about the governance of emerging technologies.
Dr. Dzau explained that the NAM launched a five-year strategic plan in 2018 that prioritized responsible innovation and endeavored to provide dynamic leadership that would proactively address these challenges (NAM, 2018). To further this objective, Dr. Dzau noted, the NAM established the Committee on Emerging Science, Technology, and Innovation in Health and Medicine (CESTI) in 2019 (NAM, 2020). In 2022, Dr. Dzau explained, the NAM extended CESTI’s work through a consensus study with the National Academies of Sciences, Engineering, and Medicine (the National Academies) that focused “on the concept of equity from among the broader set of individual- and collective-level ethical principles identified by CESTI” (NASEM and NAM, 2023). This collaboration led to the release of a 2023 report titled Toward Equitable Innovation in Health and Medicine: A Framework, which offered recommendations to better align technology development with ethical principles and foster a culture that advances responsible innovation and fair opportunity across the innovation ecosystem (NASEM and NAM, 2023).
Dr. Mathews then reviewed CESTI’s efforts from 2018–2022, including the development of three core ethical principles to guide technological innovation: justice, fairness, and transparency. Dr. Mathews explained that case studies were developed to explore the potential ethical, legal, and economic implications of specific emerging technologies, including neurotechnology, telehealth, and regenerative medicine; consider how these technologies align with the three ethical principles; and envision how such technologies may evolve in the future (Mathews et al., 2023a; Mathews et al., 2023b; Mathews et al., 2023c).
Dr. Mathews explained that the case studies and ethical principles were ultimately synthesized into a ‘straw’ governance framework and a tool, called a heat map, to assess individual developing technologies and identify immediate and long-term areas of potential risk and benefit. She described the heat map as a versatile visual tool designed to evaluate a technology’s alignment with the three core ethical principles (see Figure 3). The heat map captures both the benefits and risks, represented as alignments and misalignments with the three ethical principles, along with the qualitative magnitude of the benefits or risks. By providing a shared understanding of potential impacts, Dr. Mathews said, the heat map can serve to inform governance discussions and decision making.
Dr. Mathews explained that the heat map is designed to evaluate a particular technology at a given stage of development, guided by example questions such as “Does the technology pose risks to an individual’s personal privacy?” or “Impact on existing economic disparities?” and specific activities for consideration. Dr. Mathews emphasized that the heat map is not a rigid tool but a customizable framework, and that the resulting assessment provides indicators of alignment or misalignment with guiding ethical principles, highlighting areas where further attention or targeted interventions may be warranted. Dr. Mathews explained that the heat map offers a snapshot of a technology’s performance at a point in time and reflects its use in a specific activity or decision process under existing governance mechanisms—including current laws, regulations, professional guidelines, and institutional policies. An innovation’s benefits and risks may become more apparent as a technology develops. In addition to studying a specific technology at a specific time, Dr. Mathews noted, the heat map also incorporates relevant stakeholders to help ensure relevance and effectiveness in guiding ethical decision making.
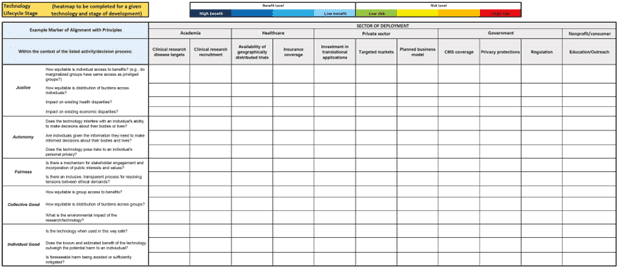
Figure 3 | The CESTI Heat Map Tool SOURCE: NASEM and NAM. 2023. Toward equitable innovation in health and medicine: A framework. Washington, DC: The National Academies Press. https://doi.org/10.17226/27184. NOTE: The heat map tool is also available online as an Excel file at https://nap.nationalacademies.org/catalog/27184/.
Dr. Wailoo then explained that CESTI’s 2023 report builds on previous NAM efforts to explore how emerging science, technology, and innovation in health and medicine can reflect ethical principles, including by highlighting equity as one of several key values guiding responsible innovation (NASEM and NAM, 2023). He noted that evidence-based investigations—offering clear methods, metrics, and benchmarks—can guide innovation, inform decision making, and support meaningful evaluation. These efforts, he added, may both advance scientific and technical excellence and help build public trust. Furthermore, Dr. Wailoo noted, addressing community health needs can increase the market reach of emerging technologies and create new opportunities for entrepreneurs, likely generating increased financial returns for investors. He noted, however, that aligning innovation with ethical values, including equity, requires a collective shift among all stakeholders.
Dr. Wailoo explained that the 2023 report outlines six key areas for action relevant to the creation of a framework for governance of emerging technologies and alignment with ethical principles, including:
- Establishing national leadership to enhance responsible innovation in science, technology, health, and medicine;
- Creating an innovation culture that integrates ethics into the organizational practice of technology development;
- Incentivizing and aligning innovation to ensure that the outputs of innovation work for everyone;
- Empowering broader participation in the innovation system;
- Developing metrics and measures for aligning technology with aims of ethical and responsible innovation; and
- Designing context-specific strategies and tools to implement across the innovation life cycle.
Dr. Wailoo stated that achieving the report’s recommendations will require coordinated actions, sustained commitments, and strong partnerships across the innovation lifecycle—including broad engagement with communities and experts in fields such as social sciences, humanities, law, and economics.
Case Studies on Emerging Technologies
In parallel breakout sessions, participants drew on ethical principles from past NAM and National Academies initiatives and utilized the CESTI heat map to analyze real-world implications of two emerging technologies: glucagon-like peptide-1 (GLP-1) agonists for weight loss and AI in drug and therapeutic development. These two examples provided contrasting thought experiments, as GLP-1 agonists represent a recently developed technology with an established market and distribution system, while AI in drug design illustrates an emerging technology that is not yet widely available to consumers. Participants explored current gaps in the development and application of these innovations, considered governance and oversight measures needed to support responsible and adaptive progress, and discussed where improved governance could prevent unintended negative impacts and broaden benefits. These discussions provided a foundation for outlining future actions—both immediate and long-term—to ensure that health and medical innovations deliver meaningful benefits to all.
Case Study: GLP-1 Agonists for Weight Loss
The session on GLP-1 agonists for weight loss, moderated by Dr. Mathews, featured a panel of experts including Dr. William Dietz, George Washington University; Mr. Joseph Nadglowski, Obesity Action Coalition; Dr. Michael Knight, IMPaCT Care and George Washington University; and Dr. Shiriki Kumanyika, University of Pennsylvania and Drexel University. This session focused on exploring the integration of ethical principles in the context of technologies with well-established markets and distribution networks.
GLP-1 agonists—a class of drugs originally developed to treat diabetes and obesity—are transforming obesity care, with current demand outpacing supply, Dr. Dietz noted. He explained that these medications work by increasing insulin secretion and reducing glucagon release, while also enhancing satiety and slowing gastric emptying. The primary drugs in this class, Semaglutide and Tirzepatide, Dietz said, differ in dosage, are approved for both diabetes and weight loss, and require gradual dose escalation and consistent use for sustained management. While these therapeutics offer weight loss rates comparable to bariatric surgery, they are costly, require weekly injections, and remain in limited supply, Dietz noted. He said that “these drugs are going to alter the entire obesity ecosystem in ways that we don’t understand,” emphasizing that the “stigmatization of obesity is pervasive and likely affects every element of care, including the development of drugs and their application.”
The panelists highlighted several challenges related to access and representation in the use of GLP-1 agonists. The panelists shared the concern that clinical trials often exclude populations at highest risk for adverse health outcomes—including individuals with severe obesity or multiple medical conditions—due to restrictive eligibility criteria, limiting understanding of how the drugs perform across certain patient groups. Dr. Knight emphasized the need for clinical trials that better reflect the populations most affected by obesity. Mr. Nadglowski echoed that the supply of GLP-1 agonists is still limited relative to demand, making it challenging to allocate doses for clinical trials. Mr. Nadglowski emphasized the importance of generating robust data about all impacts of these drugs, citing the link between rapid weight loss and increased fertility as an example of how insufficient evidence on these types of effects could pose risks to patients. Dr. Mathews emphasized the need to address such risks earlier in the research process to prevent long-term gaps in both data and clinical understanding.
The panelists also raised concerns about high cost and limited insurance coverage for GLP-1 agonists, which make them financially inaccessible for many. Mr. Nadglowski noted that insurers frequently exclude obesity medications from their formularies and that many pharmacy benefit managers add layers of complexity that drive up prices and limit access, particularly for lower-income individuals. Mr. Nadglowski also explained that stigma and social bias around drug-assisted weight loss may further discourage individuals from seeking treatment and affect how providers discuss and prescribe these medications, contributing to inconsistent and uneven care.
Dr. Dietz added that the pharmacokinetics of GLP-1 agonists vary significantly for individuals with obesity, which impacts drug effectiveness. However, he noted, these differences are often unaddressed in provider guidelines, leading to inconsistent outcomes and potential safety issues. He underscored the need to educate providers about these drugs to facilitate shared decision making and comprehensive patient support.
The panel also discussed the rapid commercialization of GLP-1 drugs through private platforms, many of which advertise compounded, unregulated versions of the medications. Mr. Nadglowski observed that patients without insurance coverage may turn to these potentially unsafe sources to access treatment. Dr. Knight stressed that societal pressures around weight loss often drive patients to seek these alternatives, underscoring the importance of avoiding patient-blaming in addressing obesity and related health impacts.
Finally, the panelists emphasized that focusing solely on medication without addressing broader environmental factors—such as access to healthy foods and the social determinants of health—fails to address the root causes of obesity, especially in historically underserved communities. Dr. Dietz suggested that educational interventions by primary care physicians could enhance shared decision making and improve patient awareness of how to use these medications effectively. Dr. Knight proposed that insurers could go further to facilitate access to effective therapeutics. Mr. Nadglowski suggested that pharmaceutical companies expand patient assistance programs to include obesity among eligible conditions. The panelists collectively advocated for a combined preventative and therapeutic approach to more comprehensively address obesity.
Case Study: Artificial Intelligence for Drug Development
The session on the use of AI tools for protein design and drug development was moderated by Dr. Tim Persons, PricewaterhouseCoopers, and featured panelists Dr. Lynda Stuart, University of Washington; Dr. Hana El-Samad, Altos Labs; Dr. Madeleine Clare Elish, Google Cloud; and Dr. Garth Graham, Google/YouTube. This session explored how ethical principles can be integrated into a foresight-oriented framework for emerging technologies that are not yet widely available to consumers.
Dr. Stuart outlined AI’s transformative potential in drug development, explaining how AI biodesign tools enable researchers to predict and engineer protein structures based on amino acid sequences or genetic codes. She noted that this capability vastly expands opportunities to design and produce drugs, vaccines, and other biotechnologies while significantly reducing development time and cost. Dr. El-Samad further emphasized AI’s role in deepening the understanding of cellular processes, highlighting how the integration of biology, engineering, and AI may foster creative exploration of complex biological systems.
As encouraged by the session, all panelists spoke about ethics and responsible use for such tools. Dr. Stuart highlighted community guidelines signed by over 200 researchers as a “playbook” for ethical AI practices (Responsible AI x Biodesign, 2024). Dr. El-Samad noted that the large-scale data generation and substantial computing power required to train AI tools create barriers to entry, effectively consolidating innovation within a few major companies and academic labs. Both Drs. Elish and Graham underscored the need to address fundamental questions around data ownership, access, transparency, and global inclusivity—particularly for researchers in the Global South. Dr. Stuart further advocated for open data access, cautioning against the concentration of proprietary information and advanced design tools within a small group of large organizations.
Dr. Graham also raised concerns about AI’s potential dual-use risks, warning that advanced tools developed for drug discovery could be misused to create harmful biological agents. He stressed the need for international collaboration and robust governance frameworks to foster responsible, beneficial use of these technologies and balance innovation with security (London, 2024a; London, 2024b). Panelists agreed that public engagement and transparency are crucial to building trust, and that partnerships among regulators, industries, and the scientific community are essential to developing practices and guidelines that address the full range of scientific, ethical, social, and biosecurity concerns raised by emerging AI technologies (Blau et al., 2024).
Inclusive governance is key to responsible AI-enabled drug development, Dr. Persons noted, referencing the Toward Equitable Innovation report (NASEM and NAM, 2023). Dr. Elish spoke to the challenge of aligning AI outputs with human values, noting that ethical alignment requires in-depth, contextualized considerations that incorporate interpretative nuance and user impact. She suggested that the health care community, with its commitment to patient-centered care, could lead by example in aligning AI with societal needs.
Case Studies: Lessons Learned
Dr. Lori Melichar, Robert Wood Johnson Foundation, and Dr. George Daley, Harvard Medical School, reconvened workshop participants to share reflections from the two case study sessions and outline key goals moving forward. Dr. Melichar emphasized the importance of developing ethical frameworks for health innovation, especially as rapid technological advancements reshape the field. She called on stakeholders across the ecosystem to play active roles in fostering responsible development. Dr. Daley highlighted the importance of proactive, transparent governance to embed these innovations within society and support broadly beneficial outcomes.
Dr. Daley noted that governance should be anticipatory—providing guidance as emerging technologies mature, rather than reacting after the fact. Reflecting on the case studies, he highlighted the health benefits of GLP-1 agonists and the promising role AI tools can play in drug and product development while stressing the need to address associated risks and the potential for unequal outcomes across patient populations. He warned that the concentration of AI technology among a few companies could widen gaps in access and opportunity. Dr. Daley concluded by underscoring the importance of balancing technological progress with safeguards against misuse, advocating for the consistent integration of ethical considerations throughout the R&D process.
Dr. Mathews reflected that the two case studies illuminated distinct challenges and opportunities. She noted that, since GLP-1 agonists are already commercially available, efforts to promote responsible use must work within an established market and distribution landscape. Dr. Mathews also identified several key levers for change, including requesting enhanced guidance from the US Food and Drug Administration, addressing societal perceptions of disease, and engaging stakeholders like primary care providers, payers, patient assistance programs, and the food industry in conversations about governance for these innovations.
In contrast, Dr. Persons observed, AI-driven drug design is still in its early stages and, as such, offers opportunities to proactively embed ethical considerations across multiple phases of development. He emphasized the need for a foresight-oriented framework that aligns innovation incentives with public interest, striking a balance between supportive guidance and overly restrictive regulation. Dr. Persons reflected that examining technologies at different stages of maturity could offer valuable insights on how ethical and inclusive approaches can be more systematically integrated across the innovation lifecycle.
Creating a Culture of Ethical and Participatory Innovation
The session on building an equitable innovation ecosystem and creating a culture of equity was moderated by Dr. Keith Yamamoto, University of California, San Francisco. Panelists included Dr. Santa Ono, University of Michigan; Dr. Patricia Mae Doykos, Bristol Meyers Squibb; Dr. Paula Hammond, Massachusetts Institute of Technology (MIT); Mr. Christopher Viehbacher, Biogen; Dr. Eric Rubin, New England Journal of Medicine; and Dr. Holden Thorp, Science journals and George Washington University. The discussion focused on integrating a range of perspectives into research and innovation systems, reshaping institutional cultures to be more open and accountable, and identifying actionable strategies to create environments that better serve patient and community needs.
Panelists agreed that transforming institutional cultures is essential to achieving better individual and population health outcomes. Dr. Thorp observed that research quality improves when faculty and trainees contribute a range of expertise and experiences. He also pointed out that researchers pursuing work in areas of systems improvement, such as workforce development or community engagement, often face barriers to workplace advancement. Dr. Rubin extended this idea to specify that broadening the research workforce should include a focus on intellectual diversity and varied lived experience—not just demographic representation.
Dr. Yamamoto discussed the importance of recognition and reward structures that value collaboration between faculty, affected communities, and patient groups in designing studies with greater real-world relevance. Dr. Ono emphasized that supporting scientific work that is tailored to patient needs requires tangible institutional support. Dr. Thorp added that the journal publication process could act as an incentive for responsible science and emphasized the need for fairer peer review processes. He cited findings from a forthcoming analysis of the Science family of journals, conducted in partnership with the University of Colorado Boulder, which found that researchers from prestigious institutions often receive more favorable reviews while others face more critical evaluations. Dr. Hammond described her efforts at MIT to build connections with community and technical colleges, broadening access to educational resources and opportunities.
The panel also addressed the role of university technology transfer offices and how they can help ensure wider innovation benefits. Dr. Ono discussed the potential for these offices to balance financial goals with strategic priorities that maximize public impact. Dr. Thorp emphasized that any changes to traditional technology transfer processes will require transparency and accountability. Dr. Hammond outlined disparities in entrepreneurship, citing a study at MIT that showed 40 percent of male faculty have founded companies, but only 22 percent of female faculty have done so (Bhatia et al., 2021). Dr. Hammond explained that programs like the Future Founders Initiative aim to close these gaps by offering trainees from underrepresented backgrounds training in entrepreneurship and access to startup funding.
Dr. Doykos added that building a stronger, more responsive innovation culture necessitates revisiting traditional approaches to research, product development, and regulatory processes. She highlighted the importance of infrastructure to support inclusion in innovation and noted that since Bristol Myers Squibb set inclusion as an institutional goal, the company has ensured that 58 percent of its clinical trials are now conducted in populations that better reflect the epidemiology of the diseases under study. Drs. Rubin and Thorp discussed how journals can contribute to stronger ethical standards: the New England Journal of Medicine, for example, now requires that clinical trial reports include participant demographic data and that such data are compared to disease prevalence to identify gaps and potential biases.
Community engagement and trust-building emerged as central themes in the panel discussion. Mr. Viehbacher shared Biogen’s approach to fostering trust with patient populations by partnering with local leaders and organizations to improve participation in clinical trials and increase the relevance of research findings. Mr. Viehbacher and Dr. Rubin both noted the importance of diverse decision makers across the public and private sectors, arguing that broader perspectives lead to better strategies for addressing the full range of community needs.
Regulatory and Funding Needs
The panel on building an equitable innovation ecosystem with a focus on funding and oversight was moderated by Dr. Gil Omenn, University of Michigan. Panelists included Ms. Holly Fernandez Lynch, University of Pennsylvania; Mr. Samsher Singh Gill, Doris Duke Foundation; Dr. Erica Kimmerling, OSTP; Dr. Susan Coller Monarez, Advanced Research Projects Agency for Health (ARPA-H); and Dr. Eliseo Pérez-Stable, National Institute on Minority Health and Health Disparities. The discussion focused on how funding strategies, governance structures, and public engagement can drive more inclusive innovation outcomes
The panelists coalesced around a central theme of the transformative role of public and philanthropic funding in reaching historically underserved populations. Philanthropic organizations, Mr. Gill argued, can take early-stage risks by investing in practices that challenge conventional models in biomedicine—often paving the way for broad institutional adoption. The panelists noted that federal agencies like the NIH and ARPA-H have also been historically positioned to close gaps in health access and outcomes through strategic resource allocation. Dr. Coller Monarez highlighted ARPA-H initiatives such as the Platform Accelerating Rural Access to Distributed and Integrated Medical Care and Health Care Rewards to Achieve Improved Outcomes, which aim to expand access to health care through new delivery models and pricing strategies designed to ensure affordability (ARPA-H, 2025a; ARPA-H, 2025b). Ms. Fernandez Lynch emphasized the need for strategic resource allocation that targets high-priority gaps in health outcomes and access.
Panelists also stressed that research design should systematically incorporate goals related to access, affordability, and population impact. Dr. Pérez-Stable shared efforts to strengthen data infrastructure to support these goals, citing tools such as the PhenX Toolkit for measuring social determinants of health and the forthcoming SCHARE platform, which will provide standardized resources to support researchers at low-resource institutions (NIMHHD, 2025; RTI International, 2025). Dr. Coller Monarez suggested that funding program managers could be tasked with evaluating projects against clear ethical criteria, including accessibility and affordability, and refining these goals over time.
Ms. Fernandez Lynch underscored the role of IRBs and regulatory bodies in reinforcing the need for ethical standards beyond basic compliance. She proposed that funders and IRBs integrate requirements for addressing access and representation into grant applications, particularly for clinical trials. However, she noted, many IRBs often lack institutional support or resources to fully support such efforts.
Ms. Fernandez Lynch also urged universities to revisit licensing practices, advocating for models that balance commercial interests with broader public access to resulting products. Collaboration between ethicists and industry, she suggested, could help maintain research integrity and prevent superficial “ethics-washing” that undermines meaningful accountability. Finally, Ms. Fernandez Lynch further recommended adjusting tenure and promotion systems to recognize work that improves public access and impact alongside traditional metrics like the number of publications and grants.
Dr. Kimmerling discussed the critical role of public engagement in shaping science policy. She described how OSTP has incorporated community listening sessions, public consultations, and formal requests for information into their policy making procedures to ensure that subsequent policies reflect the needs and priorities of a broader range of stakeholders. She stressed that ongoing public dialogue is key to building trust and embedding ethical considerations into innovation from the outset.
The panel concluded by emphasizing the need for clear definitions, benchmarks, and metrics to evaluate progress. Dr. Omenn emphasized the importance of moving beyond aspirational goals by establishing measurable outcomes related to health access, affordability, and real-world impact. He noted that philanthropic organizations, given their credibility and flexibility, are well-positioned to support this work. Dr. Pérez-Stable added that socioeconomic status—often overlooked in human subjects research—should be systematically considered alongside race, ethnicity, sex, and gender as a critical factor affecting health outcomes.
By grounding innovation initiatives in science-based measures and clear accountability frameworks, institutions can better align their work with public needs. Ultimately, the panel underscored that advancing a more inclusive innovation ecosystem will require coordinated efforts across philanthropy, government, academia, and the broader public.
Areas of Future Focus/Key Themes
Victor Dzau, National Academy of Medicine; Hana El-Samad, Altos Labs; Juan Enriquez, Excel Venture Management; Anthony Ryan Hatch, Wesleyan University; Alex John London, Carnegie Mellon University; Shobita Parthasarathy, University of Michigan; Timothy Persons, PricewaterhouseCoopers; and Jay Schnitzer, MITRE
Based on the collective insights shared during the workshop discussions, the above-named individual authors believe that five key areas of action are most crucial to ensure the complete integration of ethical principles into the development and use of emerging technologies, including:
- Integrating Fairness and Access from the Outset: Fairness and access must be built into innovation processes from the earliest stages of research, development, and distribution—not treated as an afterthought. Organizations should adopt policies, training, and incentives that embed considerations of population needs, access, and impact throughout the innovation lifecycle. In addition, there is a need to develop an evidence-based field focused on measuring the impact of innovation across different communities. This field should establish qualitative and quantitative benchmarks to assess how innovations affect health outcomes, access, and opportunity at every stage.
- Developing a Comprehensive Governance Framework: Innovation portfolios should balance commercial success with societal benefit, and effective governance must involve collaboration across the public, private, and nonprofit sectors. A comprehensive governance model should be flexible enough to adapt to a range of emerging technologies while remaining anchored in ethical values such as fairness, inclusion, privacy, autonomy, and accountability. Cross-sectoral engagement will ensure that governance frameworks address the full spectrum of impacts associated with emerging technologies and distribute responsibilities appropriately among institutions.
- Creating Practical Tools to Assess Benefits and Risks: Operationalizing ethical governance requires tools that enable anticipatory, transparent decision making, and instruments like the CESTI heat map provide visual frameworks to evaluate a technology’s alignment with core ethical principles over time. Such tools enable stakeholders from across sectors to build a shared understanding of potential benefits, risks, and tensions as technologies mature. This shared understanding can inform governance discussions and support decision making processes. Multisectoral experts can play key roles in refining and updating the frameworks that underlie these tools, ensuring they remain relevant in dynamic contexts. Incentivizing voluntary participation from industry stakeholders is critical, as their insights will help fine-tune tools, frameworks, and recommendations. By fostering collaborative engagement, these approaches can enhance the governance of emerging technologies.
- Strengthening Public Involvement in Innovation: Public engagement must move beyond one-way communication to directly involve communities in setting research and innovation priorities. Members of affected populations should be included early in the development process to ensure that technological advancements align with real needs. Mechanisms such as citizen juries, participatory technology assessments, community advisory boards, and collaborative research partnerships can provide structured opportunities for input. Successful engagement requires ongoing relationships, transparency, and a demonstrated willingness to incorporate public feedback into decision making.
- Enhancing Accessibility and Affordability of Innovations: Access and affordability must be central considerations in biomedical innovation. New products and technologies should not be priced out of reach for large portions of the population. Policies should promote solutions that are affordable compared to existing alternatives and ensure that technological advancements expand—rather than restrict—access to care and treatment. Affordability benchmarks should be integrated into funding and evaluation processes to encourage innovation that serves a broader range of users.
Moving Toward Implementation at the National Academy of Medicine
In his closing comments, Dr. Dzau emphasized that governance of emerging technologies often occurs within isolated technology or sector-specific silos. However, he noted, advancing scientific knowledge and technologies to improve health outcomes requires an innovation ecosystem that considers incentives, benefits, and risks for all users from the outset—and continually reassesses these factors as technology develops and their broader impacts become clearer.
Dr. Dzau explained that building the right conditions for a science, technology, and innovation system that serves broad public interests will require active participation across government, nonprofits, the private sector, and the public. While foundational principles for a public-private partnership to strengthen responsible innovation have been outlined, Dr. Dzau noted that the necessary infrastructure, tools, and decision making systems still need to be developed. He also stressed that successful governance must incorporate incentives for investors and technology developers to ensure broad adoption of responsible practices. Dr. Dzau acknowledged that while actionable steps have been identified by the NAM and others, sustaining collective progress will require independent, unbiased leadership to convene a diverse range of stakeholders and support ongoing engagement.
To translate the principles outlined in the workshop into action, Dr. Dzau announced the establishment of a new NAM Action Collaborative—a public-private partnership designed to promote priority-setting, coordination, and collective action for a more responsible innovation system in health and medicine. By bringing together expertise from across scientific research, universities, funders, regulatory agencies, journals, industry, investors, and patient and community organizations, the Collaborative will provide a neutral forum for shared learning, commitment-building, and coordinated action to strengthen responsible and accessible innovation practices.
References
- ARPA-H (Advanced Research Projects Agency for Health). 2025a. HEROES: Health Care Rewards to Achieve Improved Outcomes. Available at: https://arpa-h.gov/research-and-funding/programs/heroes (accessed June 2, 2025).
- ARPA-H. 2025b. PARADIGM: Platform Accelerating Rural Access to Distributed and Integrated Medical Care. Available at: https://arpa-h.gov/research-and-funding/programs/paradigm (accessed June 2, 2025).
- Bhatia, S., N. Hopkins, S. Liou, L. Millar-Nicholson, F. Murray, D. Nelson, T. Nelson, and L. Snover. 2021. MIT women and men faculty in science and engineering as founders and board members of companies in science and technology. MIT Faculty Newsletter, March/April 2021. Available at: https://fnl.mit.edu/march-april-2021/mit-women-and-men-faculty-in-science-and-engineering-as-founders-and-board-members-of-companies-in-science-and-technology/ (accessed June 2, 2025).
- Blau, W., V. G. Cerf, J. Enriquez, J. S. Francisco, U. Gasser, M. L. Gray, M. Greaves, B. J. Grosz, K. Hall Jamieson, G. H. Haug, J. L. Hennessy, E. Horvitz, D. I. Kaiser, A. John London, R. Lovell-Badge, M. K. McNutt, M. Minow, T. M. Mitchell, S. Ness, S. Parthasarathy, S. Perlmutter, W. H. Press, J. M. Wing, and M. Witherell. 2024. Protecting scientific integrity in an age of generative AI. Proceedings of the National Academy of Sciences 121(22):e2407886121. https://doi.org/10.1073/pnas.2407886121.
- Emanuel, E. J., E. Gudbranson, J. Van Parys, M. Gørtz, J. Helgeland, and J. Skinner. 2020. Comparing health outcomes of privileged US citizens with those of average residents of other developed countries. JAMA Internal Medicine 181(3):339–344. https://doi.org/10.1001/jamainternmed.2020.7484.
- London, A. J. 2024a. Challenges to evaluating emerging technologies and the need for a justice-led approach to shaping innovation. In Realizing the promise and minimizing the perils of AI for science and the scientific community, edited by K. Hall Jamieson, W. Kearney, and A.-M. Mazza. Philadelphia, PA: University of Pennsylvania Press.
- London, A. J. 2024b. A justice-led approach to AI innovation. Issues in Science and Technology, May 21. https://doi.org/10.58875/KNRZ2697.
- Mathews, D., A. Abernethy, A. J. Butte, J. Enriquez, B. Kocher, S. H. Lisanby, T. M. Persons, R. Fabi, A. C. Offodile II, J. S. Sherkow, R. D. Sullenger, E. Freiling, and C. Balatbat. 2023a. Neurotechnology and noninvasive neuromodulation: Case study for understanding and anticipating emerging science and technology. NAM Perspectives.Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/202311c.
- Mathews, D., A. Abernethy, A. J. Butte, P. Ginsburg, B. Kocher, C. Novelli, L. Sandy, J. Smee, R. Fabi, A. C. Offodile II, J. S. Sherkow, R. D. Sullenger, E. Freiling, and C. Balatbat. 2023b. Telehealth and mobile health: Case study for understanding and anticipating emerging science and technology. NAM Perspectives.Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/202311e.
- Mathews, D., A. Abernethy, E. Chaikof, R. A. Charo, G. Q. Daley, J. Enriquez, S. Gottlieb, J. Kahn, R. D. Klausner, S. Tavazoie, R. Fabi, A. C. Offodile II, J. S. Sherkow, R. D. Sullenger, E. Freiling, and C. Balatbat. 2023c. Regenerative medicine: Case study for understanding and anticipating emerging science and technology. NAM Perspectives.Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/202311d.
- Mazzucato, M. 2013. The Entrepreneurial State: Debunking public vs. private sector myths. London, UK: Anthem Press.
- NAM (National Academy of Medicine). 2020. National Academy of Medicine announces Committee on Emerging Science, Technology, and Innovation (CESTI). NAM News, January 31. Available at: https://nam.edu/news-and-insights/national-academy-of-medicine-announces-committee-on-emerging-science-technology-and-innovation-cesti/ (accessed June 2, 2025).
- 2018. NAM Strategic Plan 2018-2023: Goalposts for a healthier future. Available at: https://nam.edu/wp-content/uploads/2017/10/National-Academy-of-Medicine-2018-2023-Strategic-Plan.pdf (accessed June 2, 2025).
- NASEM and NAM (National Academies of Sciences, Engineering, and Medicine and National Academy of Medicine). 2023. Toward equitable innovation in health and medicine: A framework. Washington, DC: The National Academies Press. https://doi.org/10.17226/27184.
- NIMHHD (National Institute on Minority Health and Health Disparities). 2025. SCHARE: Science Collaborative for Health and Artificial Intelligence Reduction of Errors. Available at: https://www.nimhd.nih.gov/resources/schare (accessed June 2, 2025).
- NSF NSB (National Science Foundation’s National Science Board). 2024. The state of U.S. science and engineering 2024. Available at: https://ncses.nsf.gov/pubs/nsb20243 (accessed June 2, 2025).
- NATO Science & Technology Organization. 2020. Science & technology trends 2020-2040: Exploring the S&T Edge. Available at: https://www.nato.int/nato_static_fl2014/assets/pdf/2020/4/pdf/190422-ST_Tech_Trends_Report_2020-2040.pdf (accessed June 2, 2025).
- OECD (Organisation for Economic Co-operation and Development). 2024. Framework for anticipatory governance of emerging technologies. https://doi.org/10.1787/0248ead5-en.
- Responsible AI x Biodesign. 2024. Community values, guiding principles, and commitments for the responsible development of AI for protein design. Responsible AI x Biodesign, March 8. Available at: https://responsiblebiodesign.ai/#preamble (accessed June 2, 2025).
- RTI International. 2025. PhenX Toolkit. Available at: https://www.phenxtoolkit.org/ (accessed June 2, 2025).
- United Health Foundation. 2014. America’s health rankings: A call to action for individuals and their communities. 25th Anniversary Edition. Available at: https://assets.americashealthrankings.org/app/uploads/americas-health-rankings-2014-edition.pdf (accessed June 2, 2025).
- US Congress. 2022. CHIPS and Science Act, R. 4346, 117th Congress. Available at: https://www.congress.gov/bill/117th-congress/house-bill/4346 (accessed June 2, 2025).
- OSTP (White House Office of Science and Technology Policy). 2022. Blueprint for an AI Bill of Rights: Making automated systems work for the American people. Available at: https://bidenwhitehouse.archives.gov/ostp/ai-bill-of-rights/ (accessed June 2, 2025).

